
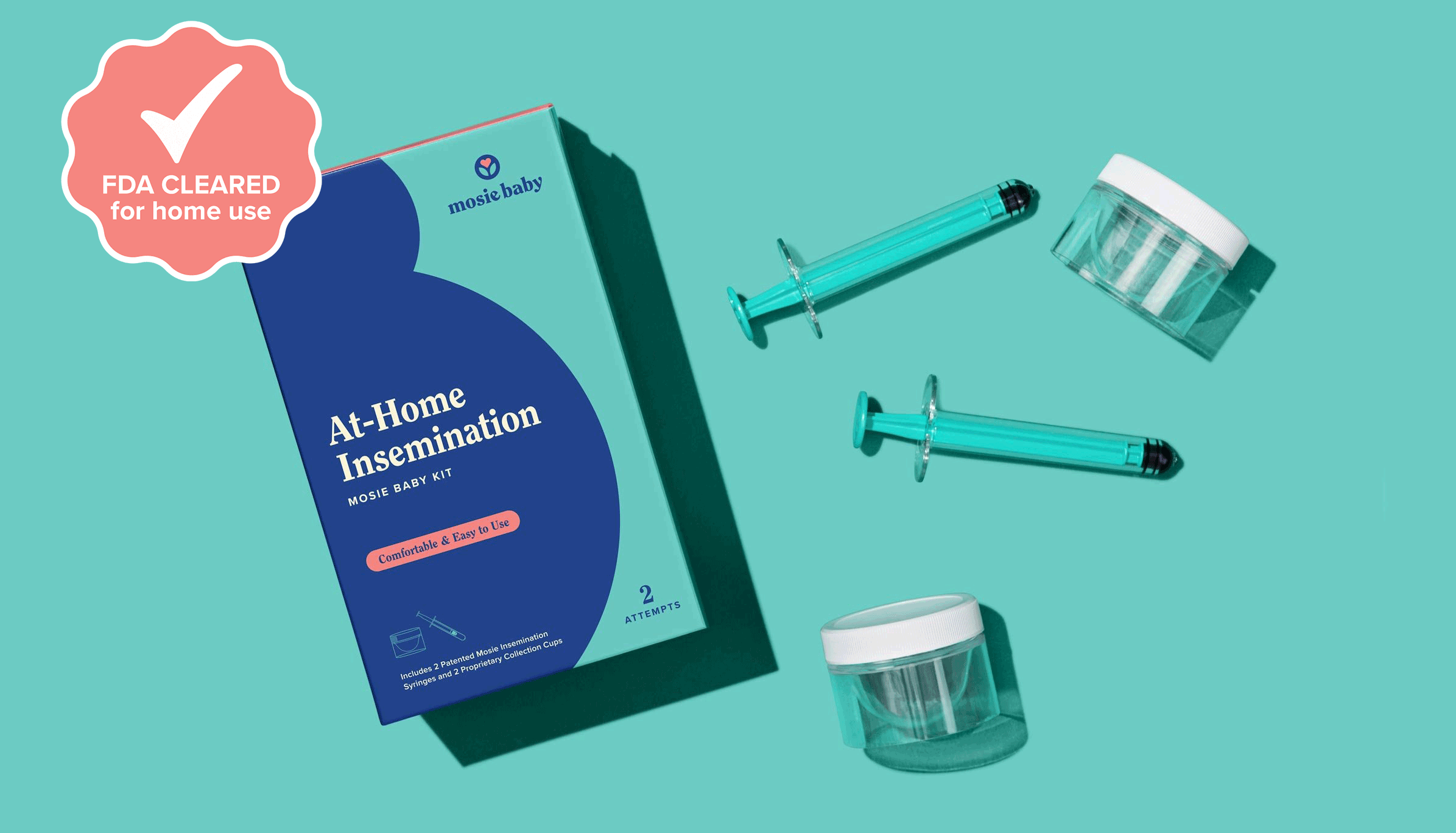
The First & Only FDA-Cleared Home Insemination Kit with a Slit Opening.
The groundbreaking Mosie Baby Kit for at-home insemination is the first intravaginal insemination kit cleared for over the counter use by the FDA. Mosie is a prescription free device for use with fresh or donor sperm when trying to conceive.
The groundbreaking Mosie Baby Kit for at-home insemination is the first intravaginal insemination kit cleared for over-the-counter use by the FDA. Mosie is a prescription-free device for use with fresh or donor sperm when trying to conceive.
Why does FDA 510(k) clearance matter?
FDA Clearance provides you with additional confidence when using the Mosie Baby Kit.
Rigorously Tested
The Mosie Baby Kit has been through a number of safety, usability, and performance tests. It was reviewed to be non-toxic to sperm*, non-irritating to vaginal tissue, and free from microbial contamination.
Built with Purpose
Mosie's patented design was created specifically for home-insemination and your body. We take extra care when manufacturing by partnering with a USA based ISO 13485 facility and each kit is clean room assembled.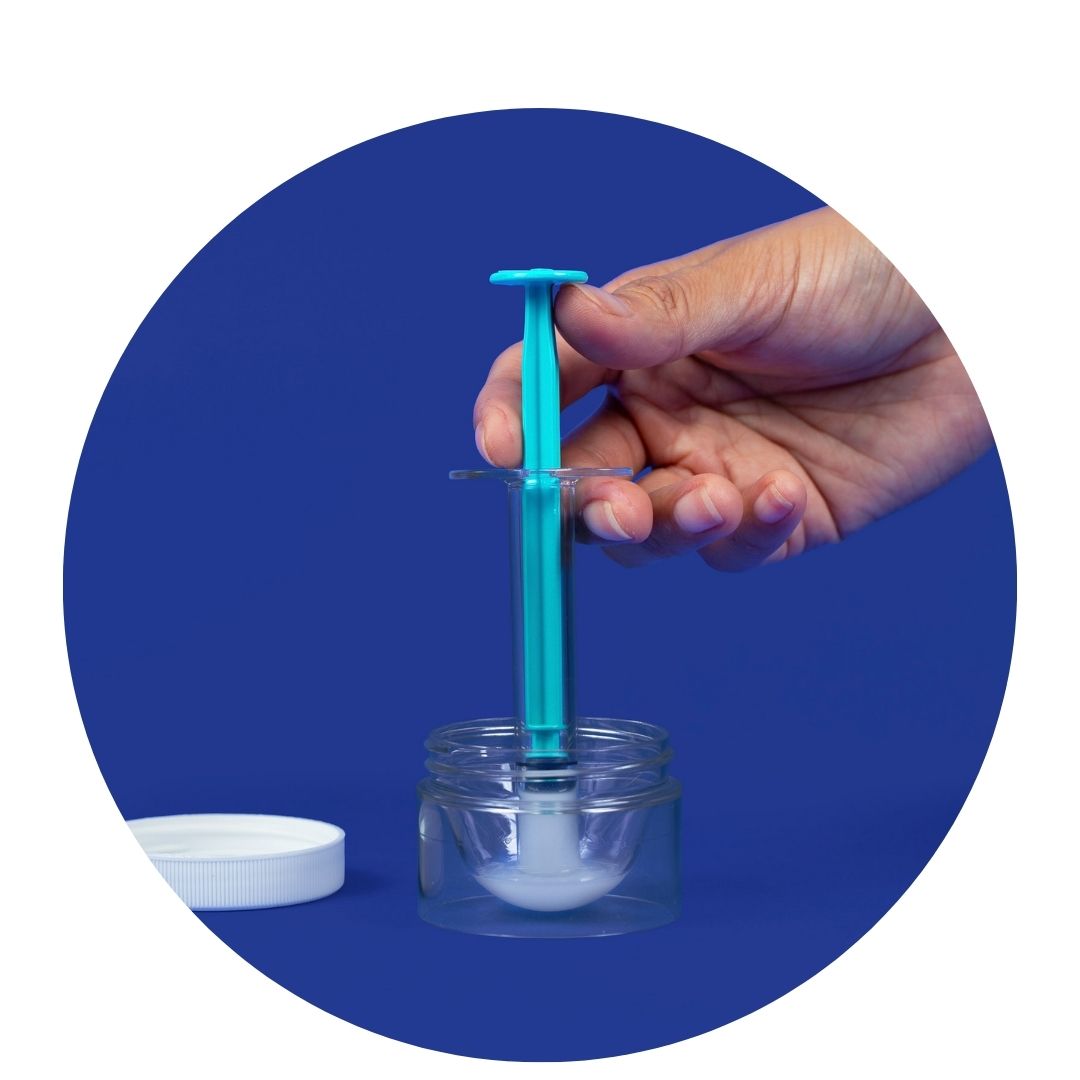
Quality Standards
We hold ourselves to the highest standards of quality. To give you peace of mind, our quality system ensures each batch of kits is tested prior to distribution. You'll notice lot codes on each kit, one of our many commitments.
What does this mean for you?
Indicated for home use when intercourse isn’t working or isn’t an option
Created by a couple who needed an alternative to intercourse, Mosie is the first FDA reviewed home intravaginal insemination kit!
Over the counter & prescription free
Mosie was designed for both comfort and ease of use, all from the comfort of your own home.

Same patented design, more confidence
Our expert recommended, patented syringe was designed specifically for insemination with a nub tip and unique slit opening that provides a deeper, wider spray.
Removed Stigma & Expanded Access
Join over 100,000 families who have trusted Mosie on their path to parenthood by shopping in stores and online
FAQ
FDA Clearance provides you with additional confidence that the Mosie Baby Kit has been extensively tested and determined by the FDA to be substantially equivalent to a device used for intrauterine insemination (IUI).
FDA Clearance is now the requirement for all devices for at-home insemination - and Mosie Baby is the first device with FDA clearance for intravaginal insemination.
Securing FDA clearance for the Mosie Baby Kit marks a pivotal milestone for the fertility community by elevating and recognizing home insemination as an important option for families, and one that requires high standards for safety and efficacy.
The FDA’s 510(k) clearance process involves a comprehensive review of safety and performance data for the device, which may include scientific, non-clinical, and clinical data, as appropriate, to determine if a new device is substantially equivalent to a device that is already on the market.
To receive FDA Clearance a device must go through the FDA’s 510(k) process where a 510(k) submission is made to the FDA to “demonstrate that the device to be marketed is as safe and effective, that is, substantially equivalent, to a legally marketed device (section 513(i)(1)(A) FD&C Act)."
The Mosie Baby Kit is indicated for over-the-counter home use, by individuals who have been unable to conceive through intercourse or choose not to conceive through intercourse, for semen collection and the delivery of semen or donor sperm to the vaginal canal. The Mosie Baby Kit should be used during the ovulatory phase of the menstrual cycle.
The term FDA Approved is used for prescription medicines and devices that are Class III, which are typically devices that are implanted or life-sustaining. Class III devices and prescription medicines require “FDA Approval” to be on the market. Home Insemination devices are Class II, and Class II devices require “FDA Clearance” to be on the market.
The Mosie Baby Kit has been through a number of safety, usability, and performance tests. These tests include rigorous clinical and technical testing which showed Mosie to be non-cytotoxic, vaginally non-irritating, non-sensitizing, not sperm-toxic (HSSA ≥ 80% of control motility after 24 hours), clean and free from microbial contamination, and that our instructions and labeling are accessible and easily understood.
Home insemination devices are not required by the FDA to be sterile. As part of our clearance process we were able to demonstrate the Mosie Baby Kit is free from harmful microbial contaminants and pathogens. The Mosie Baby Kit is also clean room assembled with multiple checks prior to being released into inventory.
Many common over-the-counter devices sold for use in the vagina are non-sterile, including tampons, condoms, and menstrual cups.
Sterilization is a process of removing microbial contaminants from a device via chemical sterilization (ethylene oxide or chlorine dioxide) or radiation sterilization. Mosie Baby avoids the need to sterilize by ensuring its devices are built clean and ready to use right out of the box.
All home insemination devices (including intravaginal syringes, cervical caps, or other devices that interact with semen and vaginal tissue) sold in the USA need to be FDA cleared through the 510(k) process in order to follow FDA regulations. The Mosie Baby Kit for at-home insemination is currently the only available intravaginal insemination kit with a slit opening on the market in the USA that is FDA Cleared.
The Mosie Baby Kit is not currently covered by any health insurance plans.
However, the Mosie Baby Kit is available for purchase with an FSA/HSA card on mosiebaby.com, at optumstore.com, or reimbursable via your FSA/HSA administrator!
Mosie Baby does partner with care organizations, benefits providers, and employers to make the Mosie Baby Kit available. If you or your employer are interested in learning more, contact us at employers@mosiebaby.com.
Many families use medicated cycles to conceive with timed intercourse. If you are using ovulation induction medications we recommend talking with your healthcare provider to see if the Mosie Baby Kit is right for you. If you would like to share more info with your doctor or if you are a medical provider, please email providers@mosiebaby.com for more information.
Get the Kit
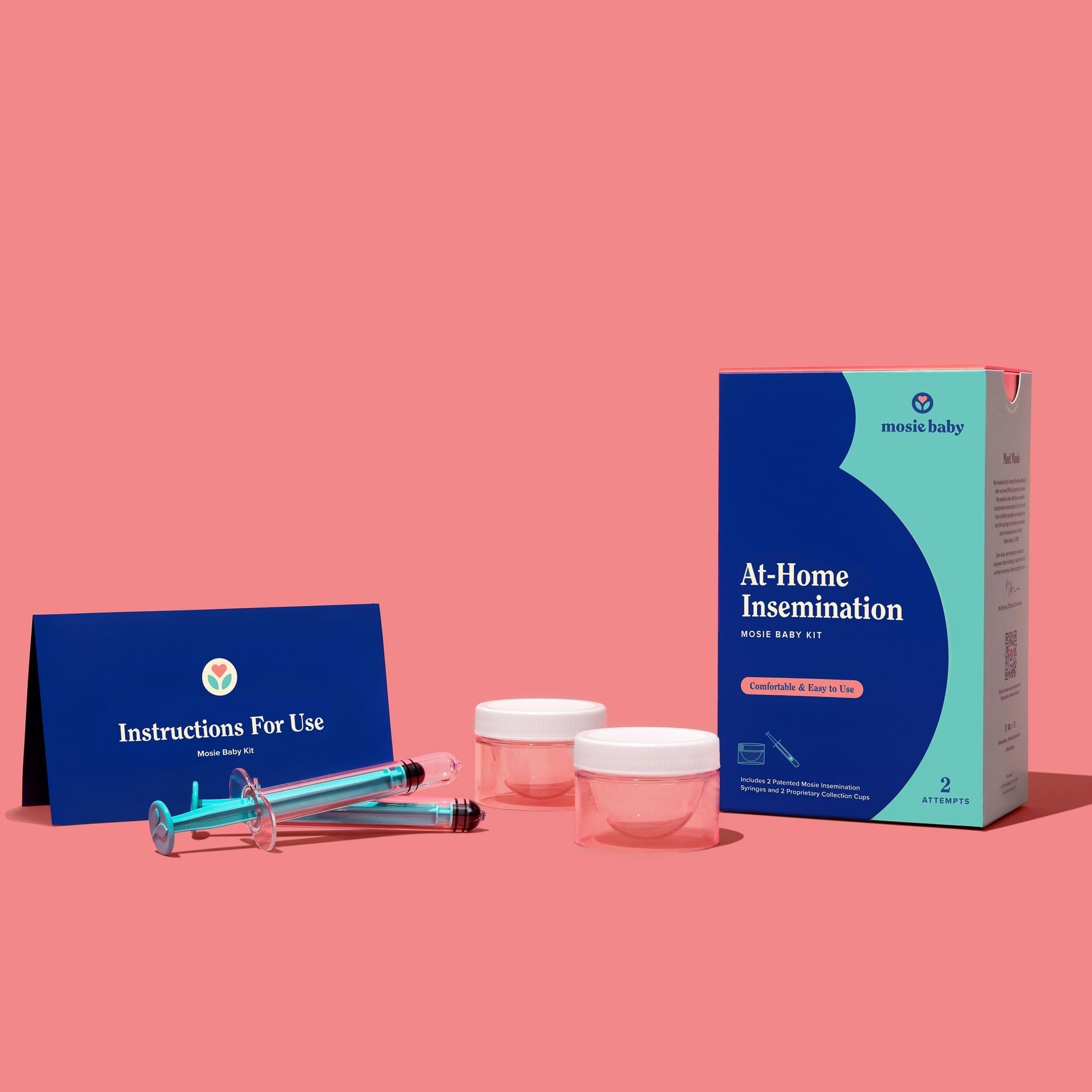
Mosie Baby Kit
Includes two patented Mosie syringes, two Mosie collection cups, and helpful instructions!







